Edge Puck™
Purpose Built for Cleanrooms
The Edge Puck is a self-contained gateway with an integrated computer that securely connects analytical instruments via Ethernet, Serial, or USB to enterprise data systems. Purpose-built for regulated manufacturing and laboratory environments, it is cleanable, portable, and fully compliant with cleanroom standards. Additional features include battery backup for data recovery and a private network architecture that eliminates communication errors from other instruments. When paired with the Phizzle Connected Plant™, the Edge Puck delivers a seamless, validated bridge between laboratory hardware and digital ecosystems such as LIMS, ELN, and MES, enabling legacy instruments to connect effortlessly to modern systems and supporting fully paperless workflows.
Request a Demo
Connecting the Edge Puck and Connected Plant

Step 1: Connect
The Edge Puck interfaces directly with analytical instruments, capturing raw sample data in real time and ensuring clean, isolated data transfer within a validated network.
Step 2: Automate
Connected Plant standardizes and structures incoming data using Phizzle’s Data Formatting Engine. Laboratory technicians and quality managers can review, approve, and e-sign results while maintaining complete audit trails for FDA and GMP compliance — all within a single paperless interface.
Step 3: Integrate
We integrate Connected Plant into your existing infrastructure and align it with current SOPs, allowing manufacturing teams to securely synchronize data with LIMS, MES, QMS, and other Digital Systems — without disrupting established workflows.
Benefits of the Edge Puck:
Extend Life of Legacy Instruments
Rather than replacing legacy instrumentation, the Edge Puck converts existing assets into connected, compliant nodes within a modern digital ecosystem. It extends the life and value of analytical instruments by enabling Ethernet connectivity and seamless digital integration — eliminating the need for costly hardware upgrades. The Edge Puck transforms legacy lab and cleanroom devices into connected data sources, bridging the gap between analog instruments and today’s digital systems.
Versatile Connectivity
The Edge Puck enables laboratory technicians to easily move and operate air particle counters (APCs), microbial air samplers, and other instruments throughout the facility — all while maintaining secure, continuous connectivity. Its compact, portable design solves the space and installation challenges common in cramped cleanroom environments, where conventional gateways are too large or impractical to deploy.
Secures Vulnerable Analytical Instruments
Many analytical instruments use exposed communication protocols that can create network security vulnerabilities. Phizzle’s Edge Puck serves as a secure gateway, converting unprotected protocols such as Modbus into encrypted MQTT communications. This architecture eliminates direct network access to instruments while maintaining full, compliant connectivity to backend systems.
Malware Hardened
The Edge Puck features a read-only file system that provides inherent protection against malware. Because nothing can be written to the operating system, malicious software cannot install, modify, or execute, ensuring a secure and stable operating environment.
Edge Puck Use-Cases:
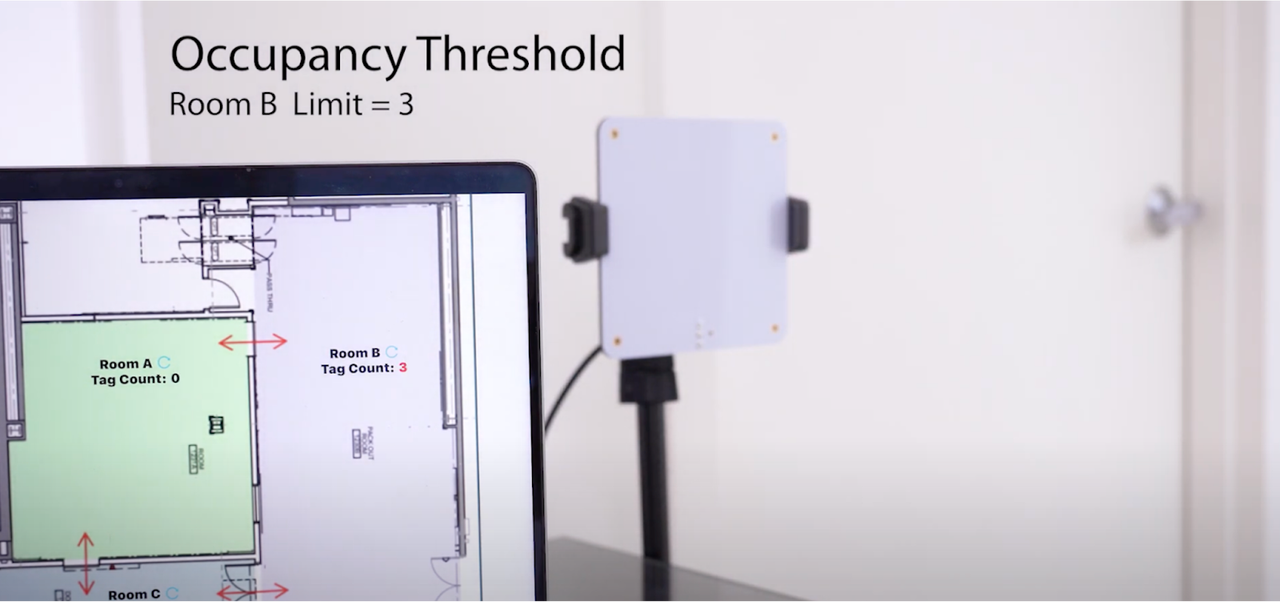
Building Automations - RFID Tracking:
Monitor cleanroom occupancy and capacity in real time with automated RFID-based tracking and alerts when thresholds are exceeded. The system maintains detailed audit trails to ensure continuous FDA inspection readiness and support for compliance reporting across regulated facilities.
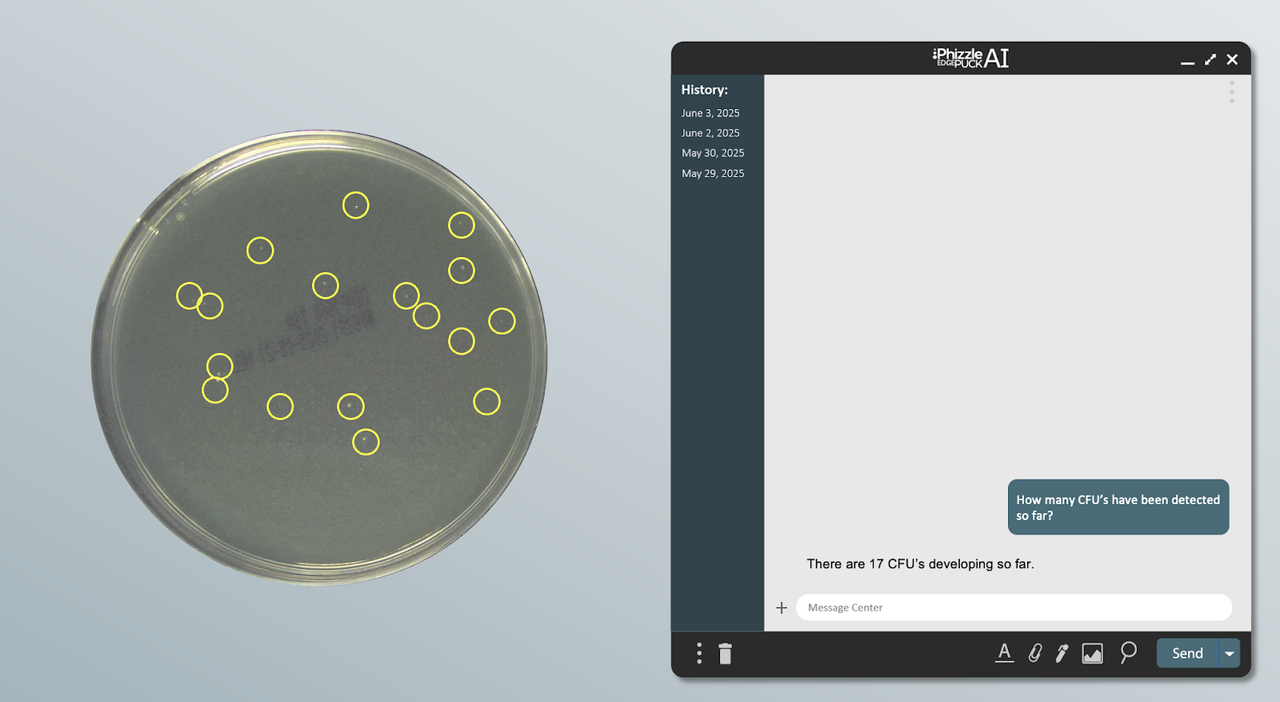
AI/ML:
Feed standardized instrument data directly into machine learning models to enable predictive maintenance, process optimization, and automated anomaly detection across manufacturing operations. By transforming raw data into structured, analyzable inputs, the Edge Puck and Connected Plant lay the foundation for AI-driven insights and continuous improvement.