Connected Plant™
for Food Production
Eliminate paper-based processes and minimize manual touch points by connecting food safety instruments directly to existing LIMS, ERP, and QMS. Accelerate production decisions by enabling real-time quality process visibility. Ensure data integrity and support HACCP/FSMA compliance with built-in electronic review, signature workflows, and continuous audit logging of operators, instruments and connected systems.
Request a Demo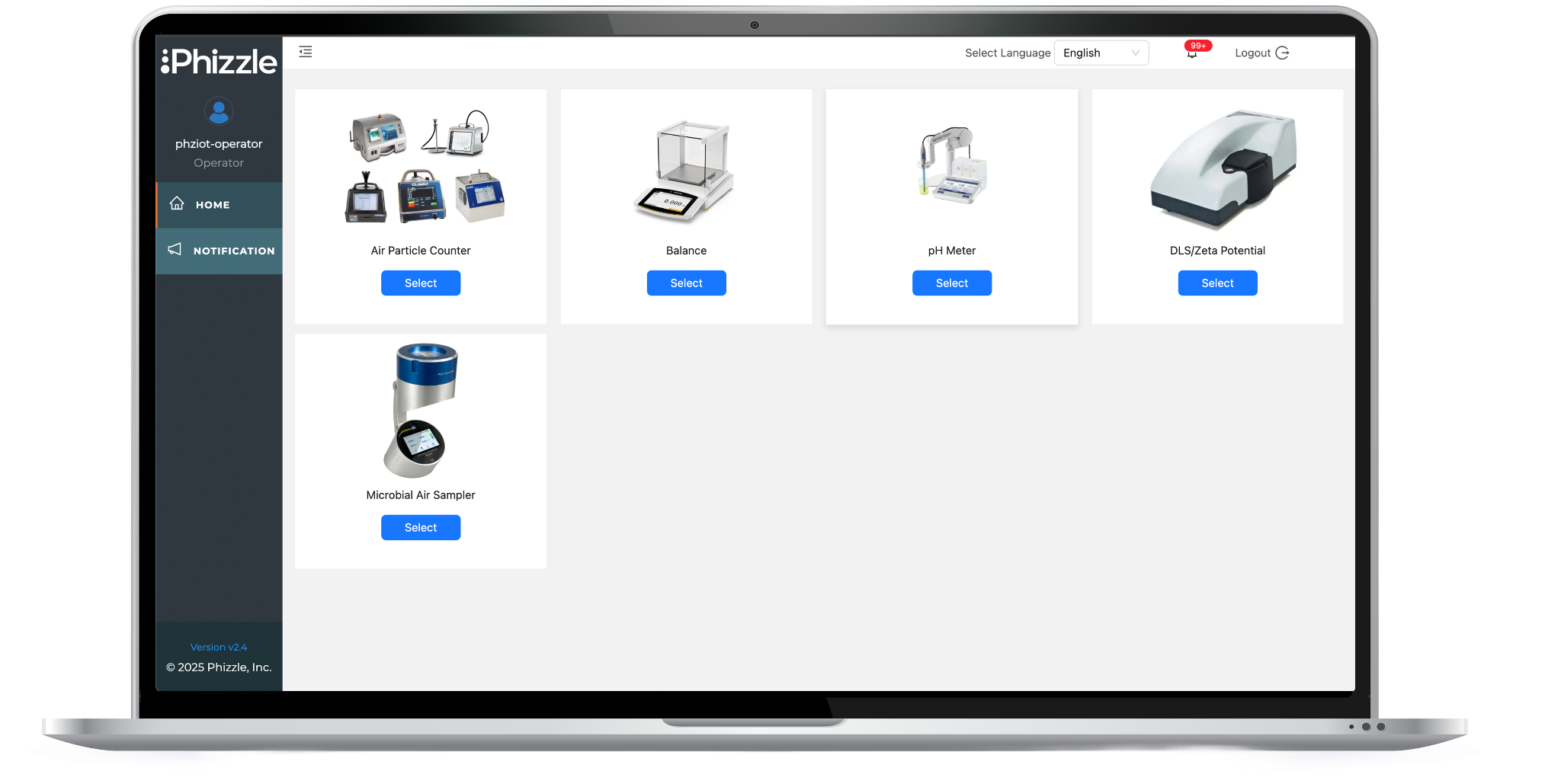
Connect Critical Food Safety Instruments to Digital Systems
Built For Critical Food Safety Instruments:
Connect with multi-vendor temperature monitoring systems, particle counters, pH meters, dissolved oxygen meters, conductivity meters, turbidity meters, moisture analyzers, water activity meters, and metal detection systems in food production and cleanroom environments.
Learn More About Connecting InstrumentsEliminate Manual Data Formatting Processes:
Automate result standardization with Our Data Formatting Engine.
Learn More About Automating Data FormattingIntegrate with Existing Digital Systems, SOPs and Compliance Parameters:
Synchronize food safety data directly with LIMS, ERP, or QMS.
Learn More About Integrating With Digital SystemsBenefits of Connected Plant in Food Production:

Ensure Data Integrity:
Ensure data integrity by integrating data sources directly with LIMS, ERP, or QMS.

Enable Analytics Foundation:
Consistent, formatted measurement data enables statistical process control, real-time trend analysis, and predictive quality capabilities. This creates the foundation for data-driven production decisions and continuous improvement initiatives.

Enhance Contamination Control:
Prevent food safety incidents, minimize corrective action investigations, and start reviewing by exception by implementing real-time monitoring and alerting for out of range continuous monitoring samples.

Streamline Compliance:
Support HACCP and FDA compliance with built-in electronic review and signature workflows that maintain documented evidence requirements. Maintain continuous inspection-readiness with organized digital records and complete audit trails of instrument data, operator actions, and system activity.