Connected Plant™
for Pharma Manufacturing
Eliminate paper-based processes and minimize manual touch points by connecting analytical instruments directly to existing LIMS, MES, and QMS. Accelerate batch disposition by enabling review by exception. Ensure data integrity and support 21 CFR Part 11 compliance with built-in electronic review, signature workflows, and complete audit trails.
Request a Demo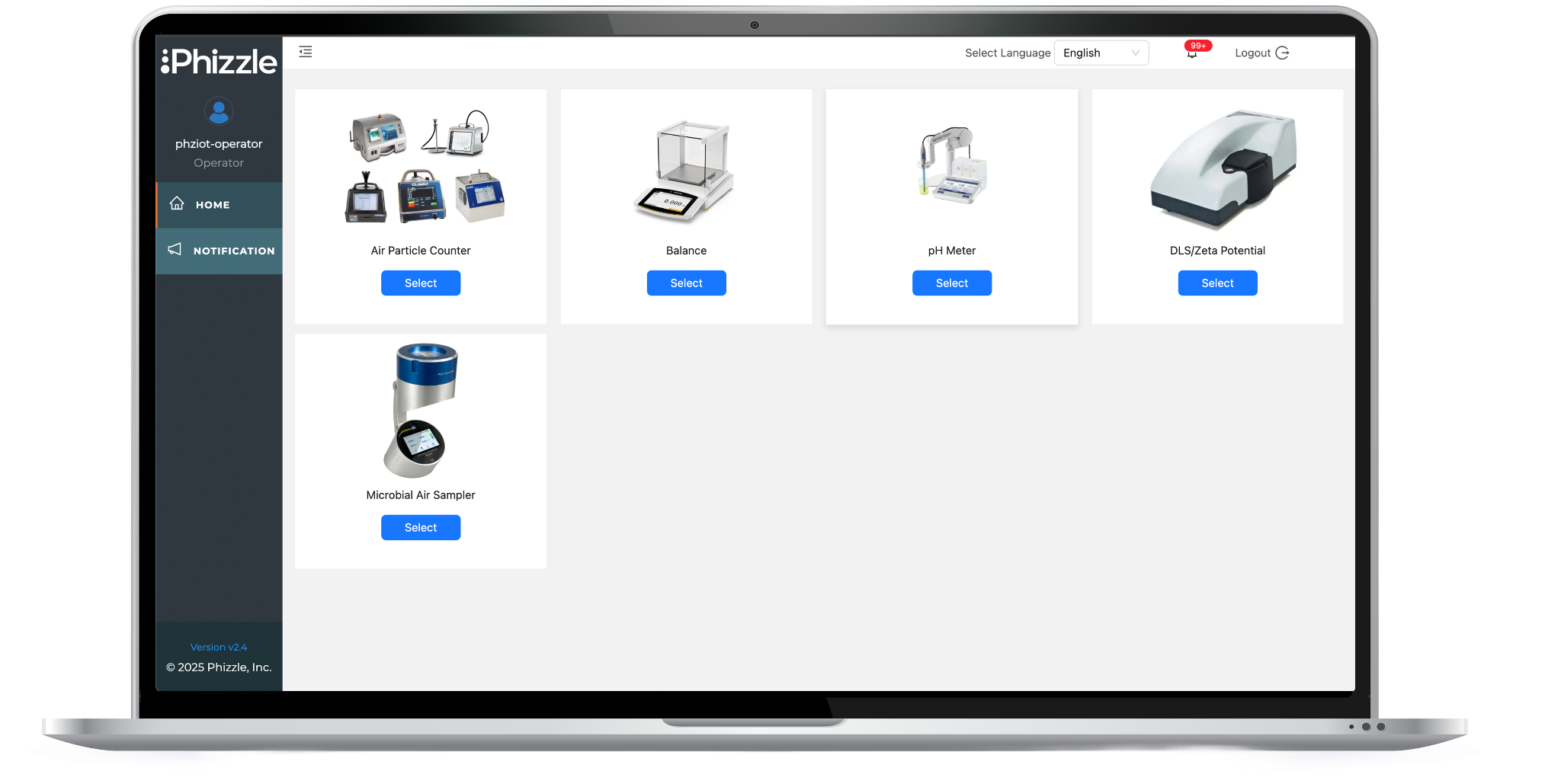
Connect GMP Instruments to Digital Systems
Built For Workhorse Instruments:
Connect multi-vendor air particle counters, microbial air samplers, pH meters, balances, zeta potential analyzers and other analytical scientific instruments in GMP/ISO environments.
Learn More About Connecting InstrumentsEliminate Manual Data Formatting Processes:
Automate result standardization with Phizzle's Data Formatting Engine.
Learn More About Automating Data FormattingIntegrate with Existing Digital Systems, SOPs and Compliance Parameters:
Synchronize critical data directly with LIMS, MES, or QMS.
Learn More About Integrating With Digital SystemsBenefits of Connected Plant in Pharma Manufacturing:

Ensure Data Integrity:
Ensure data integrity by integrating data sources directly with existing LIMS, MES, or QMS.

Reduced Cost of Quality:
Prevent deviations and minimize CAPA investigations triggered by data transcription errors and maintain continuous inspection-readiness with organized digital records and complete audit trails.

Enhanced Contamination Control:
Remote operation of particle counters and air samplers reduce personnel entry cleanroom environments. Continuous monitoring detects particle count excursions in real-time, triggering immediate investigation protocols and enabling faster corrective action before product exposure occurs.

Accelerated Batch Release:
Quality teams review by exception because out of specifications samples are caught and addressed in real-time.
See how a Top-3 Pharma is saving $1.5M a year in labor costs and cut failure reports by 80%
View the Case Study